INTRAVITAL MICROSCOPY (IVM)
is the technique of observing cellular interactions in real time.
IVM is now a recognised technique in many laboratories, it allows a qualitative and quantitative way of observing cellular interactions in real time in vivo.
Through microscopic visualisation of a tissue in an anaesthetised animal, it enables us to gather information regarding the physiological, anatomical and pharmacological mechanisms involved in cellular activity.
It was initially reported that the activated leukocyte underwent three main stages of interaction with the endothelium: rolling, adhesion and emigration, which could be quantified using IVM. However, the inflammatory cascade has been updated as understanding has developed. IVM has been key in many experiments that have led to the revisions of the inflammatory response, so that additional stages including slow rolling, firm adhesion, spreading and intraluminal crawling are now thought to be undertaken as the leukocyte interacts with the endothelium.
The use of animal models of inflammatory disease is invaluable, as the immune system is highly complex with input from not just leukocytes, but also from inside the tissue.
Without undermining the importance of in vitro models, animal models can often be the only way of gauging the response of the immune system as a whole. IVM can help to reveal these complex interactions, particularly between leukocytes and the endothelium, quantitatively and in real time, therefore allowing a better picture of how a novel anti-inflammatory agent may affect the entire system, or how effective a hypothesised target may be in mechanism of action studies.
INTRAVITAL MICROSCOPY OF LEUKOCYTE TRAFFICKING IN THE CNS
The recent advent of multiphoton microscopy and transgenic animals that express fluorescent proteins driven by tissue-specific promoters has allowed the direct observation of cell behavior under both physiological and pathological conditions in vivo.
Pioneering studies have shown that myelin-specific T cells injected in an adoptive transfer model of rat EAE crawl inside spinal cord vessels, subsequently migrating through the endothelium and displaying motility behavior, suggesting that cells resident in the CNS present antigens and activate T cells inside spinal cord parenchyma.
Two photon laser scanning microscopy (TPLSM) studies in a model of viral meningitis have also shown that myelomonocytic cells are massively recruited into the meninges during infection, leading to the loss of BBB integrity and severe seizures.
However, there have been few TPLSM studies of the CNS during inflammatory conditions and the motility behavior of leukocyte subpopulations inside CNS vessels, the transmigration mechanisms and the motility behaviour inside the parenchyma in vivo remain largely unknown.
The optical principles of two-photon microscopy are based on the absorption of two longer-wavelength lower-energy photons as a single quantum of energy by a fluorophore, thus promoting an electron to an excited state. TPLSM offers several advantages over traditional forms of microscopy for the investigation of living systems because it provides three-dimensional deep-tissue images and single-cell spatiotemporal information that other imaging techniques cannot achieve.
TPLSM is particularly suitable for high-resolution imaging in intact thick tissues such as whole organs, brain slices, embryos and live animals (intravital imaging). Extensive tissue penetration is possible due to the reduced scattering of the infrared (IR) excitation light compared to one-photon confocal microscopy. The restriction of two-photon excitation solely to the focal plane provides most of the advantages over traditional confocal microscopy. TPLSM generates fluorescence only within the focal plane, thus substantially reducing photobleaching and photodamage outside the excitation volume (which represents only a small proportion of the overall sample), and thereby prolonging the viability of specimens especially during long-term imaging.
INTRAVITAL TPLSM IMAGING IN THE CORTEX
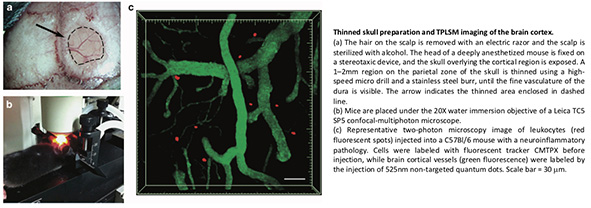
Two different surgical preparations can be used for high-resolution in vivo imaging: open cranial windows and thinned skull preparations. We and others have found that a thinned skull does not interfere with the visualization of cells up to a depth of 200 um below the pial surface. This method provides a high-resolution image of the brain tissue while maintaining normal intracerebral pressure and thus preventing mechanical injury and exposure. Where higher resolution is required, the brain cortex can be prepared for direct access by opening a 1–2 mm cranial window on the thinned skull area using a needle or forceps. Artificial CSF is applied onto the exposed cortical region. In this case, only cells lying deeper than 80 um below the pial surface should be considered for image analysis to eliminate possible artifacts caused by surgical preparation.
Long-term imaging can be achieved using mice that are deeply anesthetized, with the head fixed on a stereotaxic device. Heating is critical for the long-term maintenance of mice under anesthesia, so the core body temperature is monitored and maintained using a regulated heating pad. An incision is made along the midline of the scalp to expose the skull overlying the cortical region of interest.
Any fascia overlying the skull is scraped away with a scalpel blade. For thinned skull preparations, a 1–2 mm region on the parietal zone of the skull is thinned using a high-speed micro drill and a stainless steel burr. Drilling is interrupted every few seconds to prevent heating, and bone dust is removed using a can of compressed air. Care should be taken to avoid causing the indentation of the skull during drilling. When the fine vasculature of the dura becomes visible, thinning is continued manually using a microsurgical blade. This process is repeated until the maximum image clarity is achieved. When imaging is completed, the wound margins of the scalp are closed using a nylon suture.
For the open cranial windows procedure, a circular groove is drilled on the parietal region of the skull and the island of cranial bone is gently removed. The uncovered brain tissue is preserved using a drop of 0.9% NaCl, and when the superficial blood vessels become clearly visible a circular glass coverslip is used to cover the window and part of the skull, and is fixed in place with glue. Following surgical preparation but before image acquisition, mice are given a bolus of warm saline for rehydration and are allowed to recover from anesthesia.
Both skull preparation methods have advantages and disadvantages depending on the purpose of the investigation. The open cranial window method provides excellent optical access to the cortical layers, allowing repeated high-resolution imaging with unlimited time points and arbitrary imaging intervals. This technique is preferable for studies focusing on small structures such as dendritic spines and filopodia. However, removing the skull may induce a significant inflammatory response, involving the activation of microglia and astrocytes in the intact brain. Conversely, imaging through the thinned cranial window is a minimally invasive method, which avoids exposing the meninges and the cortex and is therefore more useful for the investigation of normal and pathological processes in the living brain. The thinned cranial preparation allows longer recording periods than an open cranial window because the thinned part of the skull protects the brain from external variations in temperature and pressure. The thinned cranial preparation is preferable for the analysis of structures larger than synaptic connections, such as the movement of leukocytes. Indeed, when following the movement of leukocytes it is important to achieve a wide observation field that allows image- acquisition periods of up to several hours. The thinned cranial preparation also allows imaging immediately after surgery.
INTRAVITAL TPLSM IN THE SPINAL CORD
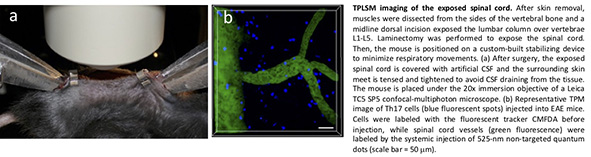
Most TPLSM studies related to spinal cord pathologies have been carried out on acute slice preparations, but the relevance of these results in vivo remains unclear. Only limited imaging work has been carried out on spinal cords in living animals. The close proximity of the heart and lungs to the spinal cord causes artifacts generated by heartbeat and breathing movements, which significantly hinders the acquisition of steady images. Here, we describe a modified method by Davalos et al. adapted for lumbar spinal cord, the main site of inflammation in mice with EAE. This new method was recently established in our laboratory and allows the stable imaging of spinal cords in mice with EAE and other spinal cord pathologies.
This method improves firmness by combining a customized spinal stabilization device with deep anesthesia to minimize respiratory movements and allow in vivo imaging without intubation or respiratory control. The mice are anesthetized with intraperitoneal ketamine/xylazine before surgery, and afterwards the spinal column is stabilized by mounting Narishige STS-A Compact Spinal Cord Clamps and a Narishige MA-6N head-holding adaptor on a steel base adapted to fit the microscope stage. After exposing the L1–L5 lumbar column by laminectomy, the muscles are dissected from both sides of the vertebral bone and the paravertebral muscles are retracted to allow the fine tips of the clamping device to be inserted. Artificial CSF is added to allow the direct immersion of the microscope objective.
Leukocytes can be labeled with vital fluorescent dyes such as CellTracker CMAC, CFSE or CMTPX, or they may express fluorescent proteins, then they are resuspended in a small volume of saline and intravenously injected into the tail vein before imaging. Alternatively, endogenous leukocytes might be labbeled by intravenous injection of fluorescent antibodies (e.g. anti-Ly6G-FITC for neutrophils), allowing the direct visualisation of the endogenous population. To image blood vessels in the cortex or spinal cord, high-molecular-weight fluorescent dextran (42000kDa) or non-targeted quantum dots are injected intravenously or retro-orbitally.
If you are curious to see some video of TPLSM in the brain of AD-like disease mice click here.