Under normal conditions, LEUKOCYTES roll along microvascular walls via low affinity interaction of selectins with specific endothelial carbohydrate ligands.
During the inflammatory response, chemotactic factors of different origin and proinflammatory cytokines signal the recruitment of leukocytes to sites of infection and/or injury. This leads to the activation of integrins and subsequent high-affinity binding to intercellular adhesion molecules on the surface of activated endothelial cells in postcapillary venules.
Under the influence of a chemotactic gradient, generated locally and by diffusion of chemoattractants from the infection/injured site, leukocyte penetrate the endothelial layer and migrate through connective tissue to sites of infection (also called diapedesis), where they finally adhere to extracellular matrix components such as laminin and fibronectin.
The step-by-step process:
The migration of leukocytes from blood vessels into the central nervous system (CNS) involves a sequence of adhesion and activation events including:
i) capture (tethering) and rolling, which are mediated by the interactions between selectins and mucins, and/or between integrins and proteins with immunoglobulin domains;
ii) activation induced by chemokines, resulting in the subsequent activation of integrins;
iii) arrest mediated by integrins and their counter-ligands;
iv) diapedesis or transmigration.
Additional steps may include slow rolling, adhesion strengthening and spreading, and intravascular crawling.
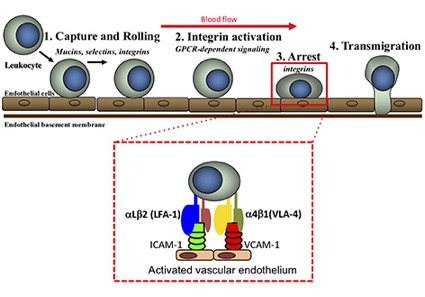
Leukocyte trafficking in the CNS during inflammatory diseases is mediated predominantly by endothelial E selectin, P-selectin and their mucin ligands, as well as leukocyte integrins including alpha4beta1 (also known as very late antigen 4, VLA-4) and alphaLbeta2 (also known as leukocyte function-associated antigen 1, LFA-1), which bind the endothelial vascular cell adhesion molecule (VCAM-1) and intercellular cell adhesion molecule (ICAM-1), respectively.
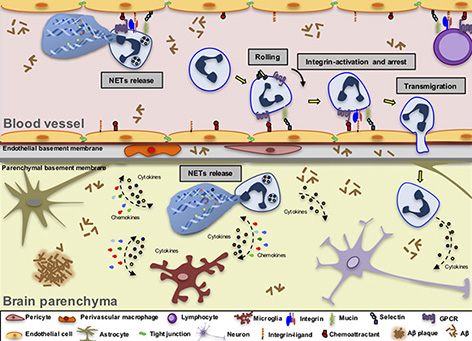
Schematic representation of leukocyte extravasation in the AD brain. This model illustrates the key steps of the leukocyte migration cascade in postcapillary brain venules, using neutrophil extravasation as an example. The activated endothelium in AD brain venules induces the expression of adhesion molecules and chemoattractants supporting the adhesion of leukocytes that may subsequently transmigrate into the brain parenchyma. The first step of the adhesion cascade is tethering and rolling, followed by a slow rolling phase mediated by endothelial selectins and mucins expressed by leukocytes. Chemoattractants such as chemokines expressed by the endothelial cells bind to leukocyte GPCRs and induce integrin activation and integrin-mediated arrest. Integrins and their endothelial ligands from the IgG superfamily also control leukocyte crawling on the endothelial cells. After crawling, leukocytes transmigrate using a paracellular route as shown here, or transmigrate through the endothelial cell body (not shown). Neutrophils that adhere to the blood vessel walls may acquire a particularly toxic phenotype and release intravascular NETs along with reactive oxygen species (ROS), cytokines, chemokines and enzymes. Leukocytes then infiltrate the brain parenchyma and become harmful to neural cells. They can release intraparenchymal NETs and ROS (in the case of neutrophils), enzymes, cytokines and chemokines, thus amplifying the activation of glial cells and causing neuronal impairment, further contributing to the exacerbation of the neuroinflammatory process in AD.
Have a look at this really nice animated movie showing Neutrophil Extravasation.