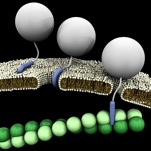
Cell-penetrating peptides (CPPs), also called trojan nano vectors or trojan peptides, are carriers for soluble, high molecular weight, molecules that are normally unable to overstep the cell plasma membrane.
Penetratin-1 (P1), from Antennapedia, and TAT, from HIV, are the best studied and characterized example of CPPs and their efficiency as systems for intracellular delivery of long peptides, entire proteins or nano particles has been widely tested for hundreds of cargos, including active proteins, and was demonstrated under several conditions, either in vitro as well as in vivo, and in different cellular contexts, including a variety of primary mammalian cells. CPPs allow the intracellular transfer of various cargoes, from small chemical molecules to nano-sized particles and large fragments of DNA, following a dose-response curve, so they display a pharmacological profile.
Because of this ability, CPPs hold great potential as in vitro and in vivo delivery vehicles for use in research and for the targeted delivery of therapeutics to individual cells. Indeed, P1 and TAT and related peptides are under extensive testing for developing novel pharmacologic approaches.
Cell-penetrating peptides are able to enter cells by means of a number of mechanisms (see review1 and review2), including direct passive entry, micropinocytosis and active endocytosis, a process by which cells actively internalize molecules by engulfing them.
CPPs are extensively developed as nanomedicine tools to deliver inside primary cells a variety of different cargos (see The Handbook of Nanomedicine). See review3 for therapeutic applications of CPP technology
Two approaches based on P1 and TAT are fully established in Laudanna’s laboratory and are routinely applied. We have successfully demonstrated the high efficiency of P1-based technique in human and murine primary leukocytes and in several signaling contexts, including signaling modulated by different PKC isoforms, various rho small GTPases, rap small GTPases, PLD1, Talin1, JAK PTKs and PTPs (PTPRG) (see refs. and ongoing projects). Notably, the P1-based approach is best suited to investigate the role of specific regulatory domains, whereas the TAT-based approach is used to deliver entire proteins (WT and mutated) into the cells. Thus, the two approaches are highly complementary.
We have ongoing projects to validate the in vivo usage of CPPs as novel therapeutical agents capable of modulating critical signaling pathways in different pathologies, such as EAE, B-CLL, extracorporeal circulation (ECC) (in collaboration with prof. G. Faggian) and atherosclerosis (in collaboration with prof. G. Constantin and prof. L. Cominacini). The projects are carried out in the context of the Nanomedicine project (WP4, coordinated by prof. L. Cominacini) (University of Verona), sponsored by Fondazione Cariverona.
Go here for the Nanomedicine European Technology Platform (ETP).
A number of P1- and TAT-based fusion CPPs have been designed and validated (see list) and are available for collaborations.
Lab experts: dr. Michela Mirenda; dr. Matteo Bolomini-Vittori; dr. Erika Lorenzetto
Published papers with CPPs technology
Bellisola G, Bolomini Vittori M, Cinque G, Dumas P, Fiorini Z, Laudanna C, Mirenda M, Sandt C, Silvestri G, Tomasello L, Vezzalini M, Wehbe K, Sorio C. Unsupervised explorative data analysis of normal human leukocytes and BCR/ABL positive leukemic cells mid-infrared spectra. Analyst. 2015 May 19. [Epub ahead of print] PubMed PMID: 25988195.
Mirenda M, Toffali L, Montresor A, Scardoni G, Sorio C and Laudanna C. Protein tyrosine phosphatase, receptor type, gamma (PTPRG) is a JAK phosphatase and negatively regulates leukocyte integrin activation. Journal of Immunology 2015 Mar 1;194(5):2168-79 PubMed PMID: 25624455.
Montresor A, Bolomini-Vittori M, Toffali L, Rossi B, Constantin G, Laudanna C. JAK tyrosine kinases promote hierarchical activation of Rho and Rap modules of integrin activation. J Cell Biol. 2013 Dec 23;203(6):1003-19. doi: 10.1083/jcb. 01303067. PubMed PMID: 24368807.
Lorenzetto E, Ettorre M, Pontelli V, Bolomini-vittori M, Bolognin S, Zorzan S, Laudanna C, Buffelli M. Rac1 selective activation improves retina ganglion cellsurvival and regeneration. PLoS One. 2013 May 29;8(5):e64350. doi: 10.1371/journal.pone.0064350. Print 2013. PubMed PMID: 23734197; PubMed Central PMCID: PMC3667179.
Rougerie P, Largeteau Q, Megrelis L, Carrette F, Lejeune T, Toffali L, RossiB, Zeghouf M, Cherfils J, Constantin G, Laudanna C, Bismuth G, Mangeney M, Delon J. Fam65b is a new transcriptional target of FOXO1 that regulates RhoA signaling for T lymphocyte migration. J Immunol. 2013 Jan 15;190(2):748-55. doi:10.4049/jimmunol.1201174. Epub 2012 Dec 14. PubMed PMID: 23241886.
Graziano F, Elia C, Laudanna C, Poli G, Alfano M. Urokinase plasminogenactivator inhibits HIV virion release from macrophage-differentiated chronically infected cells via activation of RhoA and PKCε. PLoS One. 2011;6(8):e23674. doi: 10.1371/journal.pone.0023674. Epub 2011 Aug 17. PubMed PMID: 21858203; PubMedCentral PMCID: PMC3157461.
Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J,Bolomini-Vittori M, Korf-Klingebiel M, Napp LC, Hansen B, Kanwischer A, BavendiekU, Beutel G, Hapke M, Sauer MG, Laudanna C, Hogg N, Vestweber D, Wollert KC.GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011 May;17(5):581-8. doi:10.1038/nm.2354. Epub 2011 Apr 24. PubMed PMID: 21516086.
Stadtmann A, Brinkhaus L, Mueller H, Rossaint J, Bolomini-Vittori M, Bergmeier W, Van Aken H, Wagner DD, Laudanna C, Ley K, Zarbock A. Rap1a activation by CalDAG-GEFI and p38 MAPK is involved in E-selectin-dependent slow leukocyte rolling. Eur J Immunol. 2011 Jul;41(7):2074-85. doi: 10.1002/eji.201041196. Epub 2011 Jun 7. PubMed PMID: 21480213; PubMed Central PMCID: PMC3124568.
Feigelson SW, Pasvolsky R, Cemerski S, Shulman Z, Grabovsky V, Ilani T, Sagiv A, Lemaitre F, Laudanna C, Shaw AS, Alon R. Occupancy of lymphocyte LFA-1 by surface-immobilized ICAM-1 is critical for TCR- but not for chemokine-triggered LFA-1 conversion to an open headpiece high-affinity state. J Immunol. 2010 Dec 15;185(12):7394-404. doi: 10.4049/jimmunol.1002246. Epub 2010 Nov 15. PubMed PMID: 21078912.
Della Peruta M, Giagulli C, Laudanna C, Scarpa A, Sorio C. RHOA and PRKCZ control different aspects of cell motility in pancreatic cancer metastatic clones. Mol Cancer. 2010 Mar 17;9:61. doi: 10.1186/1476-4598-9-61. PubMed PMID: 20236512; PubMed Central PMCID: PMC2846889.
Montresor A, Bolomini-Vittori M, Simon SI, Rigo A, Vinante F, Laudanna C. Comparative analysis of normal versus CLL B-lymphocytes reveals patient-specific variability in signaling mechanisms controlling LFA-1 activation by chemokines. Cancer Res. 2009 Dec 15;69(24):9281-90. doi: 10.1158/0008-5472.CAN-09-2009. Epub PubMed PMID: 19934331.
Shulman Z, Shinder V, Klein E, Grabovsky V, Yeger O, Geron E, Montresor A, Bolomini-Vittori M, Feigelson SW, Kirchhausen T, Laudanna C, Shakhar G, Alon R. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity. 2009 Mar 20;30(3):384-96. doi: 10.1016/j.immuni.2008.12.020. Epub 2009 Mar 5. PubMed PMID: 19268609; PubMed Central PMCID: PMC2803105.
Bolomini-Vittori M, Montresor A, Giagulli C, Staunton D, Rossi B, Martinello M, Constantin G, Laudanna C. Regulation of conformer-specific activation of the integrin LFA-1 by a chemokine-triggered Rho signaling module. Nat Immunol. 2009 Feb;10(2):185-94. doi: 10.1038/ni.1691. Epub 2009 Jan 11. PubMed PMID: 19136961.
Pasvolsky R, Grabovsky V, Giagulli C, Shulman Z, Shamri R, Feigelson SW, Laudanna C, Alon R. RhoA is involved in LFA-1 extension triggered by CXCL12 but not in a novel outside-in LFA-1 activation facilitated by CXCL9. J Immunol. 2008 Mar 1;180(5):2815-23. PubMed PMID: 18292502.
Giagulli C, Scarpini E, Ottoboni L, Narumiya S, Butcher EC, Constantin G, Laudanna C. RhoA and zeta PKC control distinct modalities of LFA-1 activation by chemokines: critical role of LFA-1 affinity triggering in lymphocyte in vivo homing. Immunity. 2004 Jan;20(1):25-35. PubMed PMID: 14738762.
Laudanna C, Sorio C, Tecchio C, Butcher EC, Bonora A, Bassi C, Scarpa A. Motility analysis of pancreatic adenocarcinoma cells reveals a role for the atypical zeta isoform of protein kinase C in cancer cell movement. Lab Invest. 2003 Aug;83(8):1155-63. PubMed PMID: 12920244.]
Laudanna C, Mochly-Rosen D, Liron T, Constantin G, Butcher EC. Evidence of zeta protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J Biol Chem. 1998 Nov 13;273(46):30306-15. PubMed PMID: 9804792.