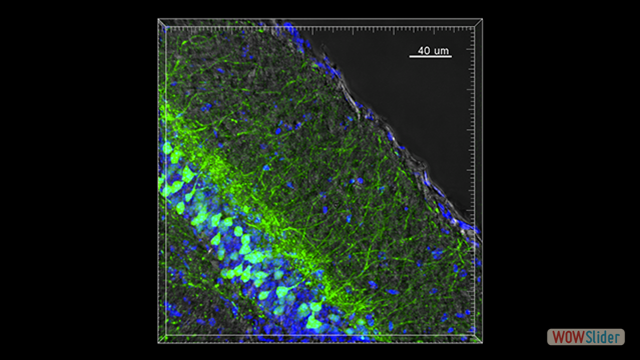
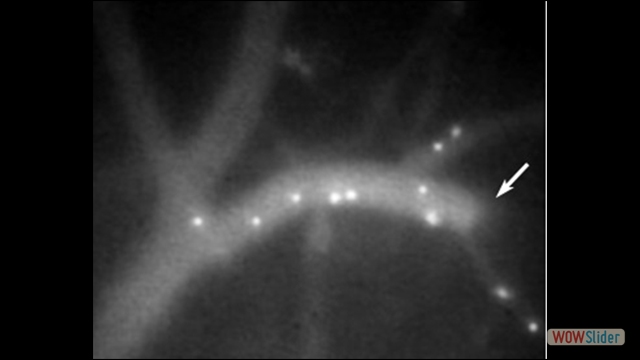
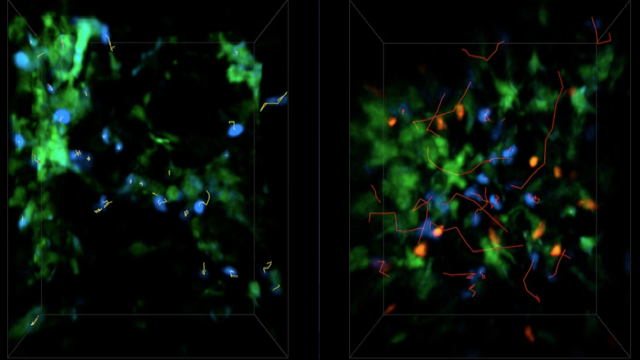
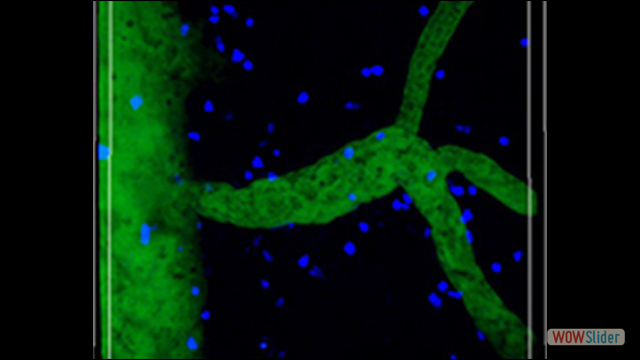
Our scientific research is focused on the fundamental aspects of innate and adaptive immune cell function, with an emphasis on the trafficking of leukocytes during autoimmune and inflammatory diseases of the central nervous system (CNS). We employ molecular and genetic approaches and intravital microscopy techniques combined with other experimental methodologies to study the migration, cell-cell interactions and function of immune cells in living animals. Our ultimate goal is to identify new therapeutic strategies for CNS diseases in which inflammation mechanisms have a detrimental role.
In the last years we identified key molecular mechanisms controlling the recruitment of leukocyte subpopulations in the CNS in animal models of multiple sclerosis, epilepsy and Alzheimer’s disease. Our recent data have shown that the T cell immunoglobulin and mucin domain 1 (TIM-1) protein is a major ligand for endothelial P-selectin, mediating Th1 and Th17 cell trafficking in inflamed tissues including the inflamed CNS during experimental autoimmune encephalomyelitis. The structural and functional features of TIM-1 show that this glycoprotein can be included in the restricted group of major adhesion receptors involved in leukocyte trafficking with a pathophysiological role in inflammation and autoimmunity.
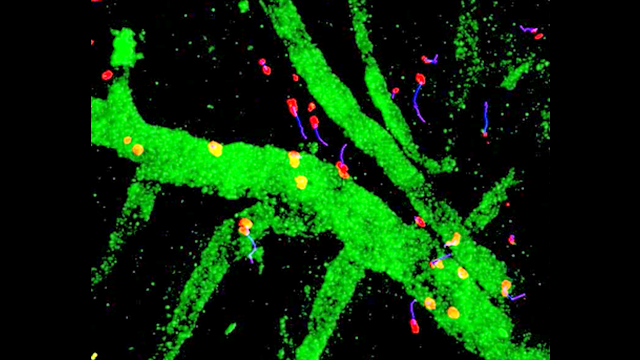
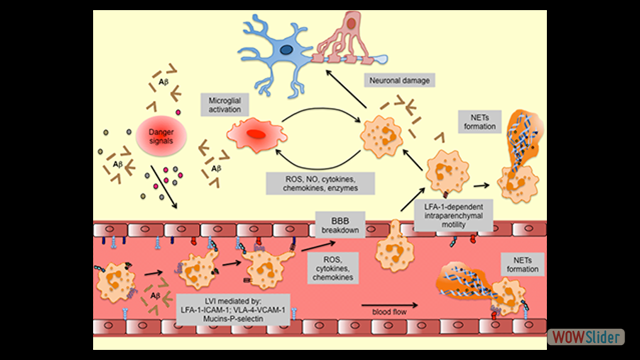
Inflammation is a pathological hallmark of Alzheimer’s disease and understanding the underlying mechanisms may facilitate the development of new treatments. We have recently shown that neutrophils invade the brain in Alzheimer’s disease-like mouse models and produce neutrophil extracellular traps (NETs) and IL-17. Neutrophil depletion and the therapeutic inhibition of neutrophil trafficking achieved by blocking LFA-1 integrin significantly reduced the memory loss and neuropathological features in mice with Alzheimer’s-like disease suggesting, that the inhibition of neutrophil trafficking may represent a new therapeutic strategy in Alzheimer’s disease. We also observed higher numbers of neutrophils and NETs in human cortical brain samples from patients with Alzheimer’s disease compared to control subjects. Our current interest is to study the role of circulating leukocytes in the induction of cognitive dysfunction and neuropathological hallmarks of Alzheimer’s disease.
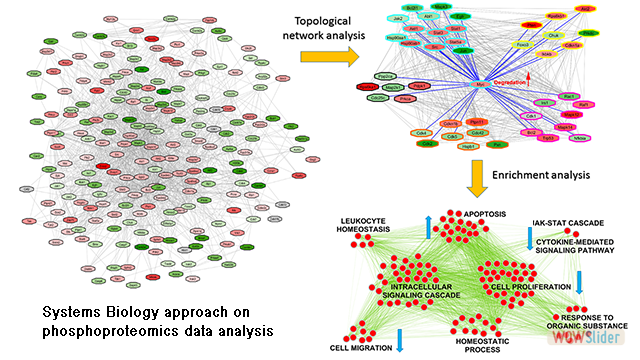
Recently, we started a novel line of research that involves data analysis, bioinformatics and systems biology. In this sense, we are developing a computational workflow that allow us modelling and analysing neuropathological data to gain a deeper knowledge of the pathological mechanisms underlying Alzheimer's disease. Major Areas of Research: Selected Publications:
Neutrophils promote Alzheimer's disease-like pathology and cognitive decline via LFA-1 integrin. A complete list of publications can be found here
Full address: University of Verona, School of Medicine,
Zenaro E, Pietronigro E, Bianca VD, Piacentino G, Marongiu L, Budui S, Turano E, Rossi B, Angiari S, Dusi S, Montresor A, Carlucci T, Nanì S, Tosadori G, Calciano L, Catalucci D, Berton G, Bonetti B, Constantin G.
Nat Med. 2015 Aug;21(8):880-6. doi: 10.1038/nm.3913. Epub 2015 Jul 27.
Regulation of T cell trafficking by the T cell immunoglobulin and mucin domain 1 glycoprotein.
Angiari S, Constantin G.
Trends Mol Med. 2014 Dec;20(12):675-84. doi: 10.1016/j.molmed.2014.10.003. Epub 2014 Oct 31.
TIM-1 glycoprotein binds the adhesion receptor P-selectin and mediates T cell trafficking during inflammation and autoimmunity.
Angiari S, Donnarumma T, Rossi B, Dusi S, Pietronigro E, Zenaro E, Della Bianca V, Toffali L, Piacentino G, Budui S, Rennert P, Xiao S, Laudanna C, Casasnovas JM, Kuchroo VK, Constantin G.
Immunity. 2014 Apr 17;40(4):542-53. doi: 10.1016/j.immuni.2014.03.004. Epub 2014 Apr 3.
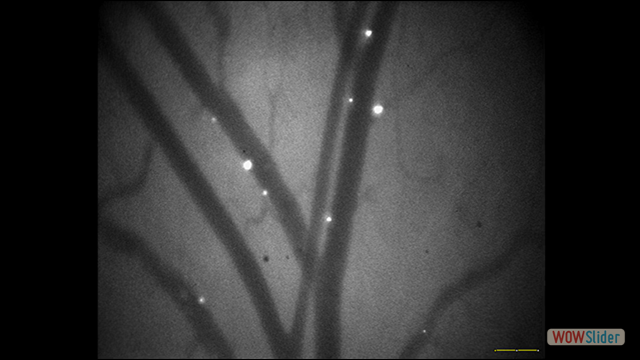
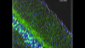
Use of imaging to study leukocyte trafficking in the central nervous system.
Zenaro E, Rossi B, Angiari S, Constantin G.
Immunol Cell Biol. 2013 Apr;91(4):271-80. doi: 10.1038/icb.2012.81. Epub 2013 Jan 22. Review.
Regulatory T cells suppress the late phase of the immune response in lymph nodes through P-selectin glycoprotein ligand-1.
Angiari S, Rossi B, Piccio L, Zinselmeyer BH, Budui S, Zenaro E, Della Bianca V, Bach SD, Scarpini E, Bolomini-Vittori M, Piacentino G, Dusi S, Laudanna C, Cross AH, Miller MJ, Constantin G.
J Immunol. 2013 Dec 1;191(11):5489-500. doi: 10.4049/jimmunol.1301235. Epub 2013 Oct 30.
A role for leukocyte-endothelial adhesion mechanisms in epilepsy.
Fabene PF, Navarro Mora G, Martinello M, Rossi B, Merigo F, Ottoboni L, Bach S, Angiari S, Benati D, Chakir A, Zanetti L, Schio F, Osculati A, Marzola P, Nicolato E, Homeister JW, Xia L, Lowe JB, McEver RP, Osculati F, Sbarbati A, Butcher EC, Constantin G.
Nat Med. 2008 Dec;14(12):1377-83. doi: 10.1038/nm.1878. Epub 2008 Nov 23.
![]()
Department of Medicine, Division of General Pathology,
Laboratory of Neuroimmunology,
Strada le Grazie 8, 37134, Verona, Italy-EU.
Phone - administrative assistant: ++39-045-8027120; Fax: ++39-045-8027127
Last update 02/01/2016

.png)

 1
1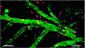 2
2 4
4 5
5 7
7 8
8 9
9 10
10